|
|
ALPHA |
BETA |
GAMMA |
|
SYMBOL |
a |
b |
c |
|
DETAILS OF EMISSION |
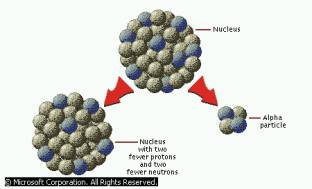
Alpha Particles are a single particle consisting of two Protons and two neutrons An Alpha Particle is identical to the nucleus of a Helium Atom |
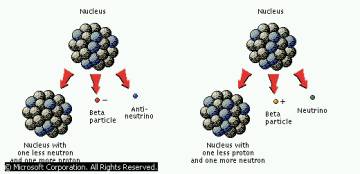
There are two types of Beta 1) A Proton turns into an Neutron by emitting an antineutrino and a NEGATIVE beta particle 2) A Neuron turns into a Proton by emitting a neutrino and a POSITIVE beta particle |
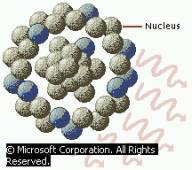
Gamma rays are high energy PHOTONS. Emitted when a radioactive nucleus decays. |
|
PENETRATION |
Poor- Only through thin Materials. Air can stop it E.g. Alpha particles from polonium-210 travel 3.8cm through air before being completely stopped |
LOW/Medium Because of the size (and the fact that Beta particles are ejected at higher speeds) Beta will penetrate considerably more matter than Alpha E>G 3 or four layers of paper/ thin Aluminum |
HIGH- Gamma rays have great penetration. In some cases they can even pass through several inches of Lead |


